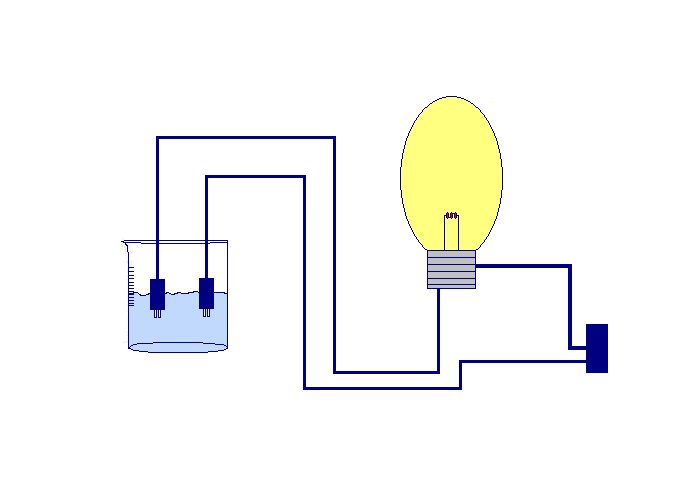
 Conductivity is the name that designates a physical property that is present in some bodies, materials or elements and that makes them capable of conducting electricity or heat through them. That is to say, those materials that conduct electricity or heat have the facility to allow the electric current to pass freely through them.
Conductivity is the name that designates a physical property that is present in some bodies, materials or elements and that makes them capable of conducting electricity or heat through them. That is to say, those materials that conduct electricity or heat have the facility to allow the electric current to pass freely through them.
Now, there are basic conditions that determine this conductive capacity and that are the molecular and atomic structure, the temperature that this body or material presents and some other particular characteristics.
Meanwhile, in terms of conductivity, they undoubtedly stand out the metals , for its high conduction of electricity thanks to its atomic structure that facilitates it.
It should be noted that the conductivity mechanism will vary in relation to the state in which the matter is present ... for example, the methodology will not be the same if it is a solid matter or, failing that, if it is a liquid.
Liquid elements have salts that are decisive in conductivity. They are found at the time of solution, generating both positive and negative ions that are responsible for transferring energy when that liquid is influenced by an electric field. Drivers in this sense are popularly known as electrolytes.
While in solid materials when they are subjected to an electric field are their bands of electrons that overlap and release energy when meeting the aforementioned field.
And when it comes to heat conduction we formally speak of Thermal conductivity. There are bodies that have a special ability to conduct heat. It basically consists of an element or substance transmitting kinetic energy (proper of its movement) from its molecules to others that are nearby but with which it is not in direct contact.